根据实时数据汇编,随着新冠肺炎大流行席卷全球,全球已有超过490万人死于该疾病,其中包括超过73.8万美国人约翰·霍普金斯大学系统科学与工程中心。
根据美国疾病控制和预防中心的数据,只有67.2%的12岁及以上的美国人完全接种了新冠肺炎疫苗美国疾病控制和预防中心。
免疫功能低下者可能需要第四剂:疾控中心
据报道,免疫力低下的人可能需要第四剂疫苗新发布的指南来自美国疾病控制和预防中心。
疾控中心表示,这些患者在接种第三剂现代疫苗或辉瑞疫苗六个月后,可能需要再注射一针。根据该机构的说法,第四剂可以是三种可用疫苗中的任何一种。
这与疾控中心之前所说的免疫功能低下的成年人是一致的。根据该机构的说法,第三针被认为是为这些患者建立疫苗接种所必需的,并且需要在六个月后进行加强。
美国食品和药物管理局小组为儿童接种绿色疫苗
食品药品监督管理局的顾问团周二投票支持辉瑞儿童疫苗5岁11岁。
十七人投了赞成票,一人弃权。
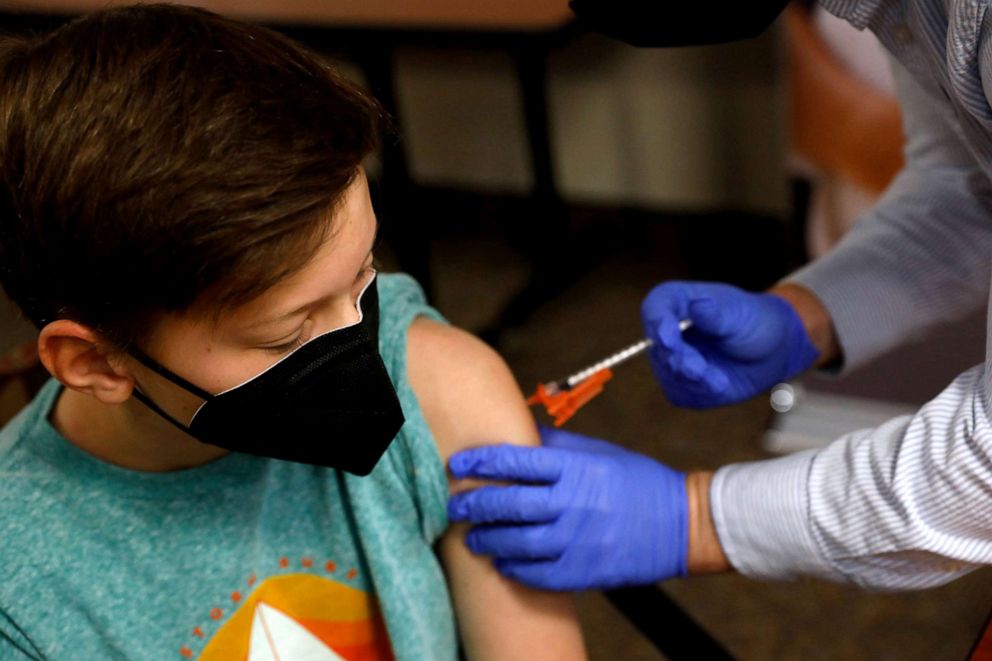
杰夫·科瓦尔斯基/法新社,通过盖蒂图像公司,文件
艾登·阿瑟从密歇根州布卢姆菲尔德山的药剂师安德鲁·麦克那里获得了辉瑞-生物泰克新冠肺炎疫苗。
接下来,FDA将做出决定。然后,此事将在下周提交给疾控中心的独立顾问小组进行审议和投票,之后,疾控中心主任预计将做出最后的签字。
最早的枪声可能是在11月的第一周。
白宫官员周二在私人电话中告诉州长,一旦食品药品监督管理局在未来几天内批准,拜登政府将开始为5至11岁的儿童运送疫苗。
这样做将允许儿童在疾病控制和预防中心签字后立即开始接受注射,预计在11月4日左右。
白宫新冠肺炎联邦反应协调员杰夫·赞茨说,一个大问题是儿科剂量的保质期缩短。为了让儿科医生更容易处理疫苗,5至11岁儿童的剂量只能保持10周,而成人的剂量则需要6至9个月。
"我们不想有浪费,所以我们鼓励你在你的分配系统中建立灵活性,你可以在你的州或地区内移动,”他告诉州长们。美国广播公司新闻获得了通话音频。”你需要什么就点什么。我们有充足的供应。我们总能在短时间内给你注射。"
食品药品监督管理局和疾病控制和预防中心的官员在周二的食品和药物管理局小组会议上解释了5到11岁的儿童是如何受到大流行影响的。官员们说,他们造成了超过190万例感染和超过8300例住院,其中约三分之一需要入住重症监护室。
官员称,该年龄组已有近100名儿童死于新冠肺炎病毒,这使该病毒成为该年龄组目前十大死因之一。
美国食品和药物管理局的独立咨询小组周二正在辩论是否批准辉瑞疫苗用于5至11岁的儿童。该小组不具约束力的投票预计将于周二晚上进行。
在专家组就是否推荐辉瑞进行投票后,FDA将做出决定。然后,此事将提交给疾控中心的独立咨询小组进行审议和投票,该小组定于11月2日和11月3日举行会议。一旦疾控中心小组投票,疾控中心主任预计将做出最后的签字。
COVID-19 live updates: Immunocompromised people may need 4th dose: CDC
As the COVID-19 pandemic has swept the globe, more than 4.9 million people have died from the disease worldwide, including over 738,000 Americans, according to real-time data compiled byJohns Hopkins University's Center for Systems Science and Engineering.
Just 67.2% of Americans ages 12 and up are fully vaccinated against COVID-19, according to data from theU.S. Centers for Disease Control and Prevention.
Immunocompromised people may need a fourth dose of the vaccine, according tonewly issued guidancefrom the U.S. Centers for Disease Control and Prevention.
Those patients may end up needing an additional shot six months after their third dose of the Moderna or Pfizer vaccines, the CDC said. The fourth dose can be of any of the three available vaccines, according to the agency.
This is in line with what the CDC has said before regarding immunocompromised adults. A third shot is considered necessary to establish vaccination for those patients and a boost would need to come six months later, according to the agency.
An advisory panel at the Food and Drug Administrationvoted Tuesday in support of the Pfizer vaccine for kids5 ages 11.
Seventeen people voted "yes" and one person abstained.
Next, the FDA will make a decision. Then, the matter heads to the CDC's independent advisory panel to deliberate and vote next week, and after that, the CDC director is expected to make the final signoff.
The earliest shots could be in arms is the first week of November.
The Biden administration will begin shipping vaccine doses for kids ages 5 to 11 as soon as the Food and Drug Administration gives the green light in coming days, White House officials told governors on a private phone call Tuesday.
Doing so will allow children to begin receiving shots as soon as the Centers for Disease Control and Prevention signs off, which is expected around Nov. 4.
Jeff Zients, the White House coordinator on the federal response to COVID-19, said one big concern is the shorter shelf life for pediatric doses.In trying to make the vaccine easier for pediatricians to handle, the doses for kids 5 to 11 can be kept for only 10 weeks, compared with six to nine months for adult doses.
"We don’t want to have wastage, so we encourage you to build flexibility into your distribution systems you can move around within your state or territory," he told the governors. Audio of the call was obtained by ABC News."Just order what you need. We have plenty of supply. We can always get you doses on short notice."
Officials with the Food and Drug Administration and Centers for Disease Control and Prevention opened Tuesday's FDA panel meeting by explaining how children 5 to 11 years old are impacted by the pandemic. They have accounted for over 1.9 million infections and over 8,300 hospitalizations, about a third of which have required ICU stays, officials said.
Nearly 100 children in that age group have died from COVID-19, making the virus one of the top 10 causes of death in this age range at this time, officials said.
The independent FDA advisory panel on Tuesday is debating whether to authorize the Pfizer vaccine for children ages 5 to 11. The panel's nonbinding vote is expected Tuesday evening.
Afterthe panel voteson whetheror not to recommend Pfizer,the FDA will make a decision. Then,the matter heads to the CDC's independent advisory panel to deliberate and vote, which is scheduled for Nov. 2 and Nov. 3. Once the CDC panel votes, the CDC director is expected to make the final signoff.






